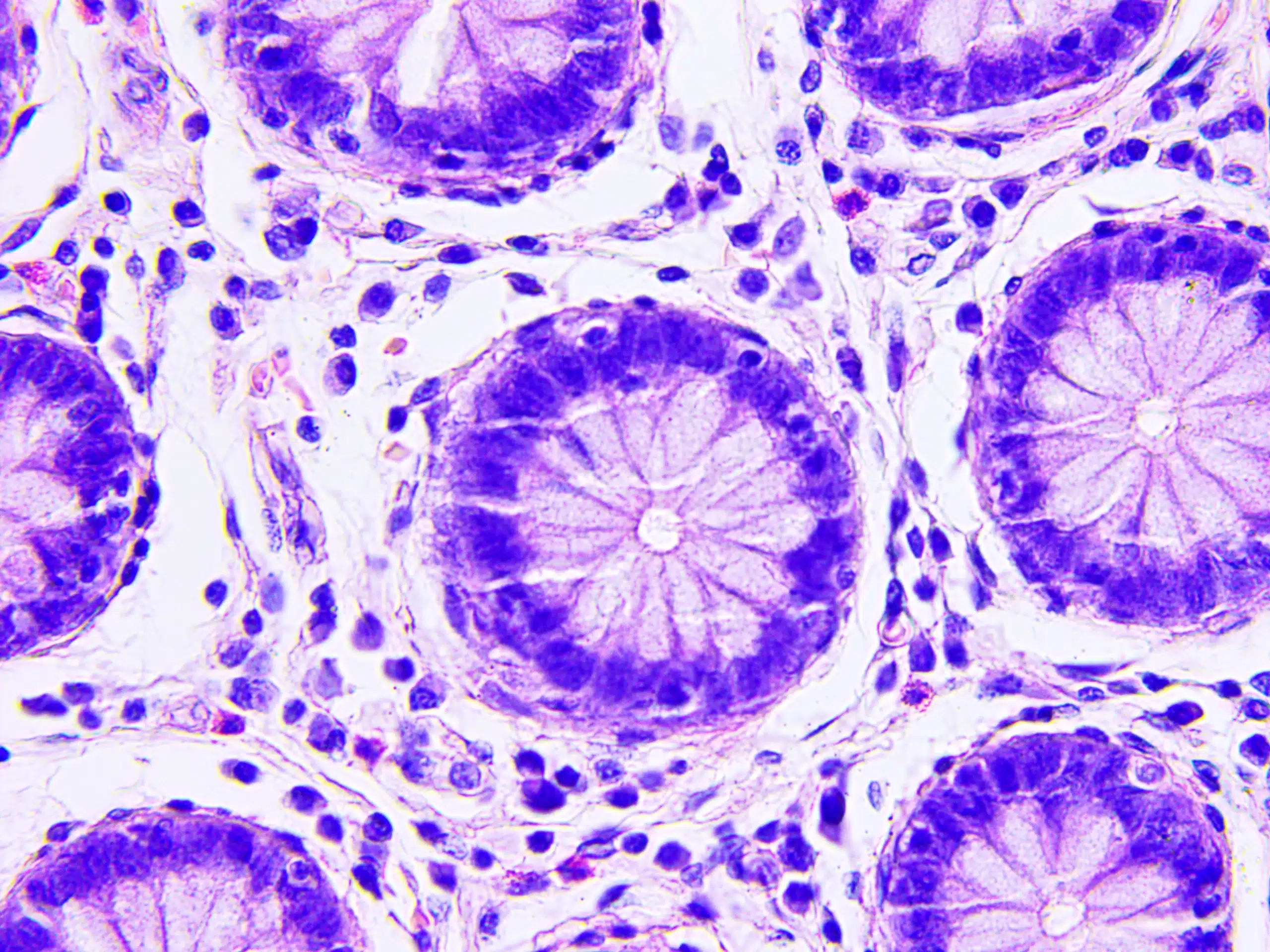
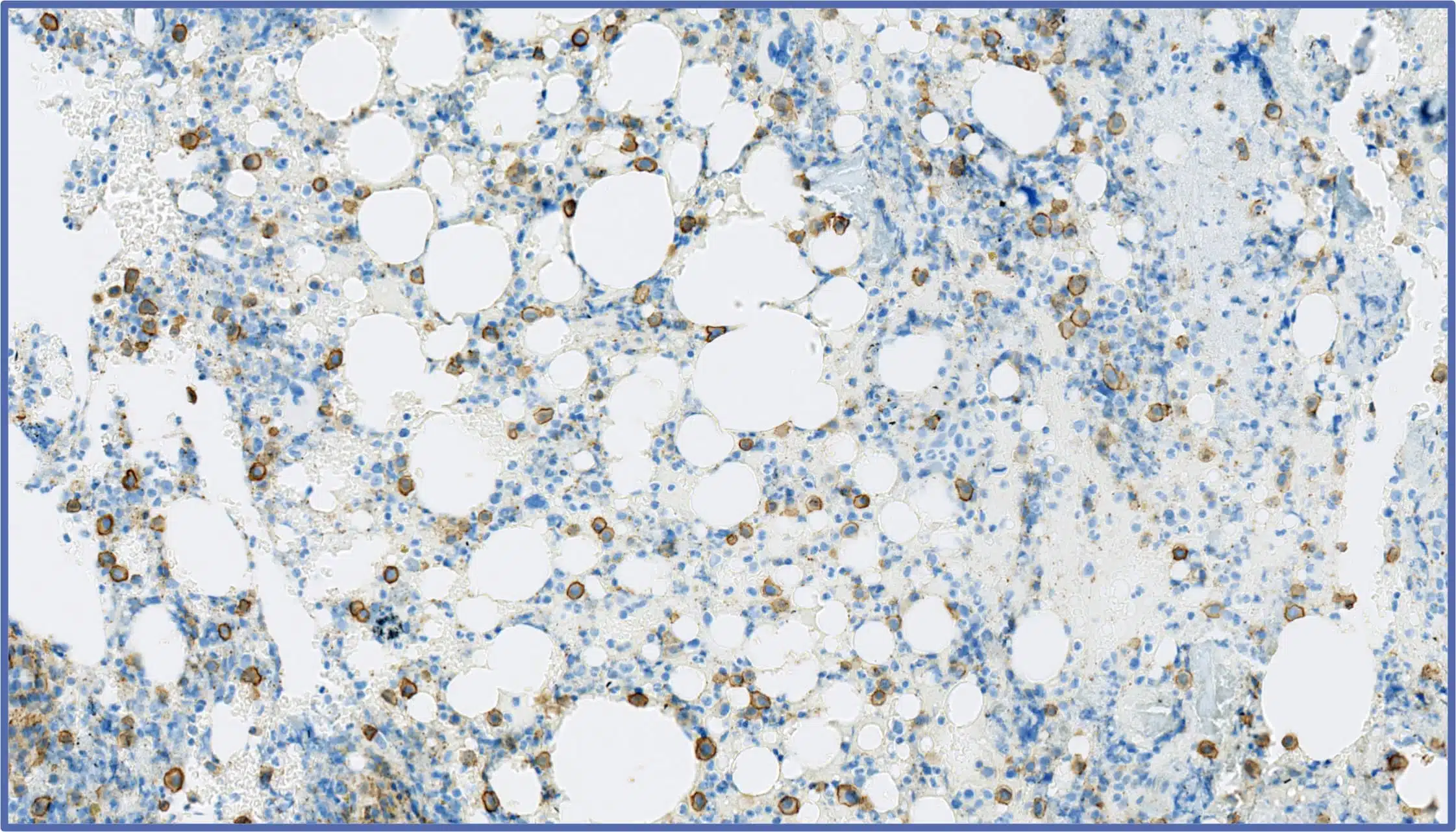
The Multiple Myeloma challenge
Multiple myeloma (MM) is a complex haematological malignancy that demands highly specialized laboratory testing to develop targeted therapies. In particular, multiple myeloma therapy development relies on precise biomarker identification, rigorous validation processes, and seamless global deployment of assays.
Our specialists support a wide range of MM studies from discovery to preclinical studies to phase III registration trials and beyond.